
New Products in Development
DrugAbuse Sciences, Inc. is engaged in the discovery and development of potential medications for the treatment of substance abuse disorders. Research and development programs focus on both the prevention of relapse as well as the treatment of overdose. Pharmacological treatment of alcohol and drug addiction has been a growing area of research, as more information about the disease process is developed. In the past, pharmacological treatment of alcoholism was initially restricted to the use of Antabuse® (a registered trademark of Wyeth-Ayerst Laboratories), a medication that causes people who drink alcohol to become nauseated. In 1994, the Food and Drug Administration approved naltrexone tablets, a medication that, when utilized within a comprehensive treatment program, reduces relapse to alcohol dependence.
Research has now established that addiction appears to be related to disruptions in the neurochemical pathways of the human body. DrugAbuse Sciences is supporting the research and development of medications that are intended to affect these pathways and in turn enhance recovery from alcohol and drug addiction.
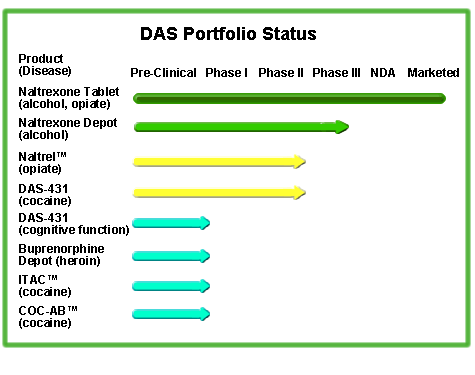
The DAS product portfolio appears below. Please browse the area below the table for a description of each product.
LACTIZ™ (extended-release drug delivery technology)
DrugAbuse Sciences has developed an extended-release injectable technology: LACTIZ™. This technology combines a drug product within a poly (D,L-lactide) polymer matrix. This polymer is biocompatible, and has been approved in other drug products as well as implantable devices. The combination of drug product with the polymer forms a dry powder of microspheres. When these microspheres are mixed with a specially formulated diluent, the product forms a suspension, which can be administered via intra-muscular injection. The drug-polymer matrix can be designed to provide for release of the drug over a period of time varying from as little as 14 days to 60 days or more. Products that are currently approved for use within the United States for treatment of either alcohol or opiate dependence generally must be taken by the patient at least once-per-day. Products that must be taken daily typically suffer from therapeutic pitfalls, especially where efficacy is predicated upon medication compliance. Where the patient either intentionally or unintentionally misses one or more dose, therapy can be rendered ineffective. Extended-release technologies typically are well accepted by patients as they offer a more convenient dosing regimen. The first three medications that have been identified by DrugAbuse Sciences for development in this LACTIZ™ platform are Naltrexone Depot, NALTREL™, and Buprenorphine Depot.
Naltrexone Depot
Naltrexone Depot is an extended-release injectable form of naltrexone, a medication that binds competitively at the opiate receptor sites in the brain. The precise mechanism of its action in the treatment of alcoholism is unknown, however the oral form of naltrexone is already approved in the United States for the treatment of alcohol dependence, when used in combination with a comprehensive treatment program, and demonstrates statistically significant reductions in relapse rates in alcoholics. A patient typically takes the tablet once-per-day. Naltrexone Depot is designed to be administered once-per-month. The drug is currently in Phase III clinical trials to evaluate its safety and efficacy.
NALTREL™ (naltrexone depot for injectable suspension)
NALTREL™ is being developed for use in the treatment of opiate dependence. Naltrexone competitively binds at the opiate receptor sites in the brain, thereby blocking the euphoric and other effects of opiates such as heroin. The oral form of naltrexone is already approved in the United States for the treatment of opiate dependence, when used in combination with a comprehensive treatment program, and demonstrates statistically significant reductions in relapse rates. NALTREL™ is designed to be administered once-per-month. Analysis of a Phase IIb clinical trial conducted by DrugAbuse Sciences has demonstrated the product’s ability to block the effects of hydromorphone, an opiate, over the six-week trial period. A Phase III trial in probationers is planned.
DAS-431™ IV (dopamine D1 receptor agonist)
In September 2000, DrugAbuse Sciences announced a worldwide licensing agreement with Abbott Laboratories, granting DAS the exclusive right to develop and market this compound for all human therapeutic indications. DAS-431™ IV (dopamine D1 receptor agonist) is a potentially beneficial pharmacotherapy for cognitive impairment in the elderly and a broad range of CNS diseases, including chronic addiction and schizophrenia. The dopamine D1 receptor is thought to play a role in mediating cognitive processes at the prefrontal cortex, particularly “working memory”. The number of D1 receptors appears to be decreased in chronic addicts, patients with schizophrenia, the elderly, and possibly patients with Parkinson’s disease or who have experienced a stroke. DAS-431 directly activates the D1 receptor in the prefrontal cortex and may be useful in treating these conditions. A Phase II study in cocaine dependent patients has been completed. Further studies in schizophrenia, in chronic addiction, and in the elderly are underway.
Buprenorphine Depot
Buprenorphine Depot is an extended-release injectable form of buprenorphine utilizing the Company’s proprietary LACTIZ™ technology. Buprenorphine is a mixed agonist-antagonist opiate. Another company has submitted a New Drug Application to the FDA for an oral tablet formulation for the treatment of opiate addiction. Buprenorphine Depot, currently in pre-clinical development, is being designed to be administered once every 4-6 weeks.
COC-AB™ (equine F(ab’)2 cocaine antibody)
DrugAbuse Sciences’ antibody antidote for cocaine overdose, COC-AB™, is intended for emergency treatment of cocaine overdose. COC-AB™ is manufactured under an exclusive agreement with Aventis Pasteur. The product is currently in pre-clinical development.
Home | About DAS | Products | Addiction FAQ | Investors | Licensing | Links | Contact | AlcoholMD









